Combustion performance of sub-nanometer Pd/S-1 catalyst for ventilation air methane
-
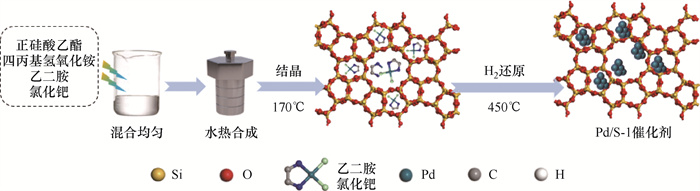
摘要: 煤矿开采导致乏风瓦斯的大量排出,不仅对环境造成较大影响,而且与我国“双碳”理念相违背。本研究通过一锅水热法制备了一种封装在Silicalite-1分子筛载体中的亚纳米Pd团簇催化剂。通过XRD、BET、SEM、XPS和TEM对催化材料的物相组成、孔结构参数、微观形貌、元素化学态以及活性组分Pd的存在状态进行表征,探讨了活性组分负载量、空速和反应温度对催化性能的影响。研究结果表明:采用一锅水热法成功将Pd团簇封装在Silicalite-1分子筛载体孔道内,制备出高分散度的亚纳米Pd团簇催化剂;随着Pd负载量的增加,催化性能呈现先升高后降低的趋势,这是由于Pd原子间产生团聚效应,导致部分Pd原子被包覆,降低了活性组分与反应物间的接触面积;以Silicalite-1分子筛为载体,可以促进活性位点骨架氧向表面羟基转化,有助于甲烷催化氧化。对比现有甲烷催化氧化工艺,本研究制备的催化剂具有催化活性高、制备方法简单、处理成本低等优点。
-
关键词:
- 甲烷催化氧化 /
- Pd团簇催化剂 /
- Silicalite-1分子筛 /
- 催化机理
Abstract: Coal mining leads to ventilation air methane emission, which not only pollutes the environment but also runs counter to "carbon neutral, carbon peaking" in China.This study prepared a sub-nanometer Pd cluster catalyst encapsulated in the Silicalite-1 molecular sieve support by the one-pot hydrothermal method, which has excellent catalytic activity and stability in methane catalytic oxidation reaction.The physical phase composition, pore structure parameters, microscopic morphology, chemical state and the present state of the active component Pd of the catalytic material were characterized by XRD, BET, SEM, XPS and TEM.This study then investigated the effects of active component loading, air velocity, reaction temperature and other conditions on the catalytic performance.The experimental results showed that the one-pot hydrothermal method successfully encapsulate Pd clusters within the pores of Silicalite-1 molecular sieve carriers to prepare highly dispersed sub-nanometer Pd cluster catalysts; the catalytic performance first increases and then decreases with the increase of Pd loading.This was due to the agglomeration effect between Pd atoms, resulting in the encapsulation of some Pd atoms and reducing the contact area between the active component and the reactants; in addition, using Silicalite-1 molecular sieve as a carrier can promote the conversion of active site skeletal oxygen to surface hydroxyl groups, which contributes to the catalytic oxidation of methane.The catalyst in this study excels existing methane catalytic process for its high catalytic activity, simple preparation, and low processing cost. -
表 1 催化实验条件
序号 甲烷浓度/% Pd负载量/% 空速/(mL·g-1·h-1) 催化燃烧温度/℃ 1 1 0,0.5,0.75,1 50 000 200~500 2 1 0,0.5,0.75,1 80 000 200~500 3 1 0,0.5,0.75,1 100 000 200~500 4 1 0.75 50 000,80 000,100 000 200~500 表 2 不同Pd负载量在不同空速下的甲烷转化温度
Table 2. Methane conversion temperature with different Pd loading at different airspeed
空速/(mL·g-1·h-1) 0.5% Pd/S-1 0.75% Pd/S-1 1% Pd/S-1 T50%/℃ T90%/℃ T50%/℃ T90%/℃ T50%/℃ T90%/℃ 50 000 350 400 320 350 320 380 80 000 355 415 320 350 325 395 100 000 360 460 325 370 325 390 -
[1] 曹敏敏, 王雪峰, 王荀, 等. 煤矿低浓度甲烷利用技术研究进展[J]. 煤炭技术, 2022, 41(1): 101-105. doi: 10.13301/j.cnki.ct.2022.01.023Cao Minmin, Wang Xuefeng, Wang Xun, et al. Research progress on utilization technology of low concentration methane in coal mines[J]. Coal Technology, 2022, 41(1): 101-105. doi: 10.13301/j.cnki.ct.2022.01.023 [2] 陈晓迈, 石雪风, 刘美茵, 等. 封装型Pd@S-1催化剂中Pd状态对低浓度甲烷催化氧化性能的影响[J]. 环境科学学报, 2023, 43(3): 394-404. doi: 10.13671/j.hjkxxb.2022.0229Chen Xiaomai, Shi Xuefeng, Liu Meiyin, et al. The effect of Pd state of encapsulated Pd@S-1 catalyst on the catalytic oxidation performance of low concentration methane[J]. Acta Scientiae Circumstantiae, 2023, 43(3): 394-404. doi: 10.13671/j.hjkxxb.2022.0229 [3] 王鹏飞, 冯涛, 陈丽娟, 等. 煤矿乏风低浓度甲烷催化氧化数值模拟[J]. 环境工程, 2012, 30(3): 67-71. doi: 10.13205/j.hjgc.2012.03.010Wang Pengfei, Feng Tao, Chen Lijuan, et al. Numerical simulation of catalytic oxidation of coal mine ventilation air low concentriation methane[J]. Environmental Engineering, 2012, 30(3): 67-71. doi: 10.13205/j.hjgc.2012.03.010 [4] Mahara Y, Ohyama J, Tojo T, et al. Enhanced activity for methane combustion over a Pd/Co/Al2O3 catalyst prepared by a galvanic deposition method[J]. Catalysis Science & Technology, 2016, 6(13): 4773-4776. [5] 袁善良, 兰海, 薄其飞, 等. TiO2掺杂CuMnCe/Al2O3催化剂对甲烷催化燃烧脱氧反应的影响[J]. 燃料化学学报, 2017, 45(2): 243-248. doi: 10.3969/j.issn.0253-2409.2017.02.015Yuan Shanliang, Lan Hai, Bo Qifei, et al. Effect of TiO2 doping on methane catalytic combustion deoxidation of CuMnCe/Al2O3 catalyst[J]. Journal of Fuel Chemistry and Technology, 2017, 45(2): 243-248. doi: 10.3969/j.issn.0253-2409.2017.02.015 [6] Yang N T, Ren Z L, Yang C G, et al. Direct oxidation of CH4 to HCOOH over extra-framework stabilized Fe@MFI catalyst at low temperature[J]. Fuel, 2021, 305(6): 121624. [7] Bu X Y, Ran J Y, Niu J T, et al. Reaction mechanism insights into CH4 catalytic oxidation on Pt13 cluster: a DFT study[J]. Molecular Catalysis, 2021, 515: 111891. doi: 10.1016/j.mcat.2021.111891 [8] Gao M Y, Gong Z M, Weng X F, et al. Methane combustion over palladium catalyst within the confined space of MFI zeolite[J]. Chinese Journal of Catalysis, 2021, 42(10): 1689-1699. doi: 10.1016/S1872-2067(20)63775-5 [9] Niu R Y, Liu P C, Li W, et al. High performance for oxidation of low-concentration methane using ultra-low Pd in silicalite-1 zeolite[J]. Microporous and Mesoporous Materials, 2019, 284: 235-240. doi: 10.1016/j.micromeso.2019.04.044 [10] Murata K, Mahara Y, Ohyama J, et al. The metal-support interaction concerning the particle size effect of Pd/Al2O3 on methane combustion[J]. Angewandte Chemie: International Ed in English, 2017, 56(50): 15993-15997. doi: 10.1002/anie.201709124 [11] 王智辉. 用于甲烷催化燃烧的金属氧化物及贵金属催化剂制备、表征及性能研究[D]. 广州: 华南理工大学, 2014. [12] Polfus J M, Løvvik O M, Bredesen R, et al. Hydrogen induced vacancy clustering and void formation mechanisms at grain boundaries in palladium[J]. Acta Materialia, 2020, 195: 708-719. doi: 10.1016/j.actamat.2020.06.007 [13] Morales-García Á, Rubeš M, Nachtigall P. The interaction of Pd clusters with the bulk and layered two-dimensional Silicalite-1 supports[J]. Catalysis Today, 2016, 277: 108-117. doi: 10.1016/j.cattod.2016.01.008 [14] Xiao C, Yang Y, Meng D, et al. Stable and active monolithic palladium catalyst for catalytic oxidation of methane using nanozeolite silicalite-1 coating on cordierite[J]. Applied Catalysis A: General, 2017, 531: 197-202. doi: 10.1016/j.apcata.2016.11.004 [15] Zhang Z S, Sun L W, Hu X F, et al. Anti-sintering Pd@silicalite-1 for methane combustion: effects of the moisture and SO2[J]. Applied Surface Science, 2019, 494: 1044-1054. doi: 10.1016/j.apsusc.2019.07.252 [16] Peng H G, Dong T, Yang S Y, et al. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes[J]. Nature Communications, 2022, 13(1): 1-10. doi: 10.1038/s41467-021-27699-2 [17] Xue W J, Mei D H. Mechanistic understanding of methane combustion over H-SSZ-13 zeolite encapsulated palladium nanocluster catalysts[J]. Chemical Engineering Journal, 2022, 444: 136671. doi: 10.1016/j.cej.2022.136671 [18] Yang L J, Cheng C Q, Zhang X, et al. Dual-site collaboration boosts electrochemical nitrogen reduction on Ru-S-C single-atom catalyst[J]. Chinese Journal of Catalysis, 2022, 43(12): 3177-3186. doi: 10.1016/S1872-2067(22)64136-6 [19] Guo Y, Liu J W, Yang Q, et al. Regulating nitrogenous adsorption and desorption on Pd clusters by the acetylene linkages of hydrogen substituted graphdiyne for efficient electrocatalytic ammonia synthesis[J]. Nano Energy, 2021, 86: 106099. doi: 10.1016/j.nanoen.2021.106099 [20] Petrov A W, Ferri D, Kröcher O, et al. Design of stable palladium-based zeolite catalysts for complete methane oxidation by postsynthesis zeolite modification[J]. ACS Catalysis, 2019, 9(3): 2303-2312. [21] Zhang Y H, Cai Y F, Guo Y, et al. The effects of the Pd chemical state on the activity of Pd/Al2O3 catalysts in CO oxidation[J]. Catalysis Science & Technology, 2014, 4(11): 3973-3980. [22] Shen Y X, Lu G Z, Guo Y, et al. Study on the catalytic reaction mechanism of low temperature oxidation of CO over Pd-Cu-Clx/Al2O3 catalyst[J]. Catalysis Today, 2011, 175(1): 558-567. [23] Wen B, Sun Q, Sachtler W M H. Function of Pdn0 clusters, Pd2+(oxo-) ions, and PdO clusters in the catalytic reduction of NO with methane over Pd/MFI catalysts[J]. Journal of Catalysis, 2001, 204(2): 314-323. [24] Monai M, Montini T, Gorte R J, et al. Catalytic oxidation of methane: Pd and beyond[J]. European Journal of Inorganic Chemistry, 2018(25): 2884-2893. [25] Guo T Y, Du J P, Wu J T, et al. Structure and kinetic investigations of surface-stepped CeO2-supported Pd catalysts for low-concentration methane oxidation[J]. Chemical Engineering Journal, 2016, 306: 745-753. -





 下载:
下载: