Mechanisms of hydration inhibition on the surface of montmorillonite in deep shale via molecular dynamic simulation
-
摘要: 井壁失稳是油气勘探开发过程中最为复杂的技术难题之一,黏土矿物水化膨胀是造成井壁失稳的关键因素,其中表面水化因水化势较大而难以去除。本文通过分子动力学模拟探测了无机盐对蒙脱石表面水化的抑制效果,以及浓度、温度和压强对水化抑制效果的影响,揭示了无机盐CaCl2抑制蒙脱石表面水化的微观机理。研究表明:抑制阳离子通过束缚蒙脱石表面水分子和降低水分子输运传导能力,从而调控水分子侵入蒙脱石表面来实现抑制作用。无机盐抑制表面水化能力依次为CaCl2>NaCl>MgCl2>KCl,钙离子易吸附表面水分子形成稳定的外球络合结构。随着CaCl2浓度增加,钙离子配位数、水化数和水化半径均降低,抑制能力减弱;温度升高和压强降低时,体系中水分子传导输运能力增强、钙离子水化数减小且力学强度降低。Abstract: Borehole instability is one of the most complicated technical problems in oil and gas exploration and development.The hydration and expansion of clay minerals is the critical factor causing wellbore instability in which the surface hydration is difficult to be removed due to the large hydration potential.In this light, through molecular dynamic simulation, this paper probed into the CaCl2 inhibitory effect of concentrations, temperatures and pressures on the surface hydration of montmorillonite which revealed the macroscopic mechanism.Results indicated that inhibition of cations were achieved by binding water molecules on the surface of montmorillonite and decreasing the transport and conductivity of water molecules, thereby regulating the invasion of water molecules into the surface of montmorillonite.The ability of inorganic salts to inhibit surface hydration were CaCl2>NaCl>MgCl2>KCl.The study found that calcium ions easily adsorbed surface water molecules to form stable outer sphere complex.With the increase of CaCl2 concentration, coordination number, hydration number and hydration radius of calcium ion decreased, and the inhibitory effect diminished.When the temperature increased and the pressure decreased, the conductivity and transport capacity of water molecules was enhanced in the system, the coordination number of calcium ion descended, and mechanical strength declined.
-
Key words:
- montmorillonite /
- inorganic salts /
- surface hydration /
- inhibition mechanism /
- molecular dynamics
-
表 1 脱水蒙脱石弹性常数与实验值和模拟值对比
Table 1. Elastic constants of dehydrated montmorillonite compared to experimentat and simulation values
Cij 弹性常数/GPa 本文值 模拟值[18] 实验值[25] C11 255.181 272.3 181.0±1.2 C22 334.081 323.6 178.4±1.3 C33 50.821 -7.2 58.6±0.6 C44 18.989 -2.3 16.5±0.6 C55 31.793 -6.2 19.5±0.5 C66 69.162 72.6 72.0±0.7 C12 123.128 129.9 48.8±2.5 C13 35.993 30.3 25.6±1.5 C14 -1.162 8.2 — C15 -66.37 -16.2 -14.2±0.8 C16 -1.020 -12.2 — C23 29.983 2.2 21.2±1.8 C24 12.563 4.1 — C25 -26.728 -8.1 1.1±3.7 C26 3.843 -3.2 — C34 0.914 -5.7 — C35 -2.114 11.9 1.0±0.6 C36 -0.225 8.8 — C45 -0.734 2.5 — C46 -14.541 2.1 -5.2±0.9 C56 -0.816 -3.1 — 表 2 脱水蒙脱石力学性质的计算结果与实验值和模拟值
Table 2. Computed results of dehydrated montmorillonite mechanical properties compared to experiment and simulation values
-
[1] Rana A, Khan I, Ali S, et al. Controlling shale swelling and fluid loss properties of water-based drilling mud via ultrasonic impregnated SWCNTs/PVP nanocomposites[J]. Energy & Fuels, 2020, 34(8): 9515-9523. [2] Anderson R L, Ratcliffe I, Greenwell H C, et al. Clay swelling—A challenge in the oilfield[J]. Earth-Science Reviews, 2010, 98(3/4): 201-216. [3] Muhammed N S, Olayiwola T, Elkatatny S. A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids[J]. Journal of Petroleum Science and Engineering, 2021, 206: 109043. doi: 10.1016/j.petrol.2021.109043 [4] Gholami R, Elochukwu H, Fakhari N, et al. A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors[J]. Earth-Science Reviews, 2018, 177: 2-13. doi: 10.1016/j.earscirev.2017.11.002 [5] 洪祥宇, 徐亨宇, 崔风路, 等. 分子模拟在非常规油气开发中的应用[J]. 计算力学学报, 2021, 38(3): 313-320. https://www.cnki.com.cn/Article/CJFDTOTAL-JSJG202103007.htmHong Xiangyu, Xu Hengyu, Cui Fenglu, et al. Application of molecular simulation in unconventional oil and gas development[J]. Chinese Journal of Computational Mechanics, 2021, 38(3): 313-320. https://www.cnki.com.cn/Article/CJFDTOTAL-JSJG202103007.htm [6] 范竞存, 余昊, 陈杰, 等. 非常规油气开采中的微纳米力学问题研究进展[J]. 中国科学技术大学学报, 2017, 47(2): 142-154. doi: 10.3969/j.issn.0253-2778.2017.02.005Fan Jingcun, Yu Hao, Chen Jie, et al. Research progress of micro/nano mechanical problems in unconventional oil and gas exploitation[J]. Journal of University of Science and Technology of China, 2017, 47(2): 142-154. doi: 10.3969/j.issn.0253-2778.2017.02.005 [7] 何满潮, 韩宗芳, 杨华. 不同温度下高岭石变形及破坏机理的分子动力学模拟[J]. 矿业科学学报, 2019, 4(1): 8-16. doi: 10.19606/j.cnki.jmst.2019.01.002He Manchao, Hanzongfang, Yang Hua. Molecular dynamics simulation of deformation and failure mechanism of kaolinite at different temperatures[J]. Journal of Mining Science and Technology, 2019, 4(1): 8-16. doi: 10.19606/j.cnki.jmst.2019.01.002 [8] Han Z F, Cui Y, Meng Q, et al. The effect of inorganic salt on the mechanical properties of montmorillonite and its mechanism: a molecular dynamics study[J]. Chemical Physics Letters, 2021, 781: 138982. doi: 10.1016/j.cplett.2021.138982 [9] Zhang Y Y, Xiao C. Molecular dynamics simulation of clay hydration inhibition of deep shale[J]. Processes, 2021, 9(6): 1069. doi: 10.3390/pr9061069 [10] Planková B, Lísal M. Molecular dynamics of aqueous salt solutions in clay nanopores under the thermodynamic conditions of hydraulic fracturing: Interplay between solution structure and molecular diffusion[J]. Fluid Phase Equilibria, 2020, 505: 112355. doi: 10.1016/j.fluid.2019.112355 [11] Svoboda M, Lísal M. Concentrated aqueous sodium chloride solution in clays at thermodynamic conditions of hydraulic fracturing: insight from molecular dynamics simulations[J]. The Journal of Chemical Physics, 2018, 148(22): 222806. doi: 10.1063/1.5017166 [12] 徐加放, 顾甜甜, 沈文丽, 等. 无机盐对蒙脱石弹性力学参数影响的分子模拟与实验研究[J]. 中国石油大学学报: 自然科学版, 2016, 40(2): 83-90. doi: 10.3969/j.issn.1673-5005.2016.02.010Xu Jiafang, Gu Tiantian, Shen Wenli, et al. Influence simulation of inorganic salts on montmorillonite elastic mechanical parameters and experimental study[J]. Journal of China University of Petroleum: Edition of Natural Science, 2016, 40(2): 83-90. doi: 10.3969/j.issn.1673-5005.2016.02.010 [13] 李小迪. 典型页岩抑制剂抑制蒙脱石水化机理的分子模拟[D]. 东营: 中国石油大学(华东), 2016. [14] 罗亚飞. Na-蒙脱石表面水化抑制机理的分子模拟[D]. 成都: 西南石油大学, 2019. [15] 谢刚. 黏土矿物表面水化抑制作用机理研究[D]. 成都: 西南石油大学, 2017. [16] Boek E S, Coveney P V, Skipper N T. Molecular modeling of clay hydration: a study of hysteresis loops in the swelling curves of sodium montmorillonites[J]. Langmuir, 1995, 11(12): 4629-4631. doi: 10.1021/la00012a008 [17] Loewenstein W. The distribution of aluminum in the tetrahedra of silicates and aluminates[J]. American Mineralogist, 1954, 39(1): 92-97. [18] Zheng Y, Zaoui A. Mechanical behavior in hydrated Na-montmorillonite clay[J]. Physica A: Statistical Mechanics and Its Applications, 2018, 505: 582-590. doi: 10.1016/j.physa.2018.03.093 [19] Cygan R T, Liang J J, Kalinichev A G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field[J]. The Journal of Physical Chemistry B, 2004, 108(4): 1255-1266. doi: 10.1021/jp0363287 [20] Al-Zaoari K, Zheng Y Y, Wei P C, et al. Early stage of swelling process of dehydrated montmorillonite through molecular dynamics simulation[J]. Materials Chemistry and Physics, 2022, 283: 126015. doi: 10.1016/j.matchemphys.2022.126015 [21] Wei P C, Zhang L L, Zheng Y Y, et al. Nanoscale friction characteristics of hydrated montmorillonites using molecular dynamics[J]. Applied Clay Science, 2021, 210: 106155. doi: 10.1016/j.clay.2021.106155 [22] 况联飞. 饱和蒙脱土高压力学特性基本机制多尺度研究[D]. 徐州: 中国矿业大学, 2013. [23] Nosé S. A unified formulation of the constant temperature molecular dynamics methods[J]. The Journal of Chemical Physics, 1984, 81(1): 511-519. doi: 10.1063/1.447334 [24] Berendsen H J C, Postma J P M, van Gunsteren W F, et al. Molecular dynamics with coupling to an external bath[J]. The Journal of Chemical Physics, 1984, 81(8): 3684-3690. doi: 10.1063/1.448118 [25] Vaughan M T, Guggenheim S. Elasticity of muscovite and its relationship to crystal structure[J]. Journal of Geophysical Research Atmospheres, 1986, 91(B5): 4657-4664. doi: 10.1029/JB091iB05p04657 [26] Hashin Z, Shtrikman S. A variational approach to the theory of the elastic behaviour of polycrystals[J]. Journal of the Mechanics and Physics of Solids, 1962, 10(4): 343-352. doi: 10.1016/0022-5096(62)90005-4 [27] Hashin Z, Shtrikman S. A variational approach to the theory of the elastic behaviour of multiphase materials[J]. Journal of the Mechanics and Physics of Solids, 1963, 11(2): 127-140. doi: 10.1016/0022-5096(63)90060-7 [28] Hill R. The elastic behaviour of a crystalline aggregate[J]. Proceedings of the Physical Society Section A, 1952, 65(5): 349-354. doi: 10.1088/0370-1298/65/5/307 [29] 张亚云, 陈勉, 邓亚, 等. 温压条件下蒙脱石水化的分子动力学模拟[J]. 硅酸盐学报, 2018, 46(10): 1489-1498. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201810022.htmZhang Yayun, Chen Mian, Deng Ya, et al. Molecular dynamics simulation of temperature and pressure effects on hydration characteristics of montmorillonites[J]. Journal of the Chinese Ceramic Society, 2018, 46(10): 1489-1498. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201810022.htm [30] 徐加放, 孙泽宁, 刘洪军, 等. 分子模拟无机盐抑制蒙脱石水化机理[J]. 石油学报, 2014, 35(2): 377-384. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201402022.htmXu Jiafang, Sun Zening, Liu Hongjun, et al. Molecular simulation for inorganic salts inhibition mechanism on montmorillonite hydration[J]. Acta Petrolei Sinica, 2014, 35(2): 377-384. https://www.cnki.com.cn/Article/CJFDTOTAL-SYXB201402022.htm [31] Zhang L H, Lu X C, Liu X D, et al. Hydration and mobility of interlayer ions of(Nax, Cay)-montmorillonite: a molecular dynamics study[J]. The Journal of Physical Chemistry C, 2014, 118(51): 29811-29821. doi: 10.1021/jp508427c [32] Sposito G, Skipper N T, Sutton R, et al. Surface geochemistry of the clay minerals[J]. PNAS, 1999, 96(7): 3358-3364. [33] 彭陈亮. 蒙脱石界面水化及疏水调控机理的量子力学/分子动力学研究[D]. 淮南: 安徽理工大学, 2016. [34] Li X, Zhu C, Jia Z Q, et al. Confinement effects and mechanistic aspects for montmorillonite nanopores[J]. Journal of Colloid and Interface Science, 2018, 523: 18-26. [35] Yu H, Xu H Y, Fan J C, et al. Transport of shale gas in microporous/nanoporous media: molecular to pore-scale simulations[J]. Energy & Fuels, 2021, 35(2): 911-943. -
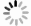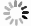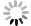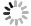





 下载:
下载: