Study on the properties of steel slag used as sulfur fixation agent in coal burning
-
摘要: 为研究燃煤固硫过程中钢渣的固硫性能,分析了固硫过程的工艺参数钙硫比、空气流量、炉温、炉内停留时间等单因素对固硫率的影响,并设计正交实验分析各因素对固硫率主次顺序的影响,确定了最优实验参数。使用X射线荧光光谱仪(XRF)分析了灰渣的结渣性和钢渣的固硫效果;应用X射线衍射光谱仪(XRD)表征了钢渣与固硫灰渣的衍射峰,进一步反映了钢渣的固硫性能;采用扫描电镜(SEM)表征了固硫灰渣的显微形貌,比较了钢渣固硫前后的表面结构和微观形貌,验证了其他表征结果的合理性。结果表明,钢渣在900 ℃高温下具有较好的固硫性能,固硫率达到63.17%,加入2%碳酸钠助剂后钢渣的固硫率可以达到70.08%。Abstract: In order to research the sulfur fixation performance of steel slag in the coal-fired sulfur fixation process, the effect of single parameters such as calcium-sulfur ratio, air flow rate, furnace temperature, and furnace residence time on the sulfur fixation efficiency was studied. In addition, orthogonal experiments were constructed to assess the effect of each variable on the main and secondary order of sulfur fixation efficiency, and the ideal experiment design was identified. Utilizing XRF, the slagging features of the ash and the sulfur-fixing effect of the steel slag were analyzed.XRD was utilized to characterize the diffraction peaks of steel slag and sulfur fixation slag, which indicated the steel slag's capacity to fixate sulfur. SEM was used to describe the microscopic morphology of sulfur fixing ash, and the surface structure and microscopic morphology of steel slag before and after sulfur fixing were compared to evaluate the validity of other characterisation results. The results suggest that the slag has a good sulfur fixation capacity at 900 ℃, with a sulfur fixation efficiency of 63.17%, and that the sulfur fixation efficiency of the slag may reach 70.08% when 2% Na2CO3 is added.
-
Key words:
- steel slag /
- sulfur fixing agen /
- sulfur fixation rate /
- the process parameters /
- orthogonal experiments
-
表 1 太原煤的工业分析与热值
Table 1. Analysis of proximate and heating value of Taiyuan coal samples
Mad/% Aad/% Vad/% FCad/% St,ad/% Qb,ad/(MJ·kg-1) 1.380 36.42 25.99 36.26 2.440 20.19 表 2 钢渣的化学元素分析
Table 2. Analysis of chemical element in steel slag
% 元素 Ca Fe Si Mg Mn Al P Ti V Cr 含量 30.6 17.7 7.85 2.12 2.66 1.67 0.815 0.838 0.272 0.304 元素 Na Ba K Sr Cl W Ag Zn Nb Zr 含量 0.147 0.107 0.064 0.040 0.030 0.021 0.022 0.018 0.011 0.011 表 3 正交实验因素和水平
Table 3. Factors and levels of orthogonal experiments
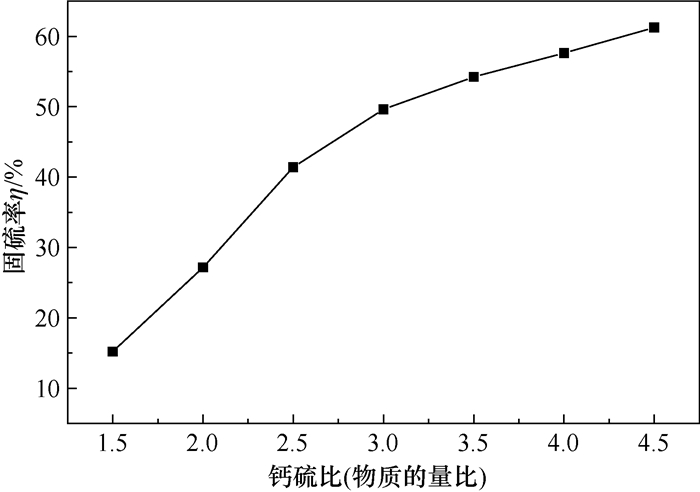
水平 钙硫比(A) 空气流量/(L·min-1)(B) 停留时间/min(C) 炉温/℃(D) 1 1.5 1 10 900 2 2.0 2 15 950 3 2.5 3 20 1 000 4 3.0 4 25 1 050 表 4 正交实验方案与结果分析
Table 4. Orthogonal experiments plan and experiment results
实验号 A B C D 固硫率/% 1 1 1 1 1 20.88 2 1 2 2 2 27.43 3 1 3 3 3 19.63 4 1 4 4 4 15.29 5 2 1 2 3 26.11 6 2 2 1 4 47.07 7 2 3 4 1 44.34 8 2 4 3 2 39.58 9 3 1 3 4 20.30 10 3 2 4 3 37.70 11 3 3 1 2 42.48 12 3 4 2 1 49.17 13 4 1 4 2 54.36 14 4 2 3 1 60.40 15 4 3 2 4 28.46 16 4 4 1 3 45.97 k1 20.807 30.412 39.100 43.697 k2 39.275 43.150 32.793 40.962 k3 37.412 33.727 34.977 32.352 k4 47.297 37.502 37.922 27.780 极差R 26.490 12.738 6.307 15.910 主次顺序 A>D>B>C 最佳方案 A4B2C1D1 注:ki为平均响应值(本实验中为固硫率),即相同因素下同一水平的实验响应值的算术平均值;R为相同因素下ki的最大值与最小值的差,R越大,表明因素的水平变化对实验的影响越大。 表 5 煤灰组成的XRF分析结果
Table 5. XRF analytical results on coal ash
% 灰样 wSiO2 wCaO wSO3 wAl2O3 wFe2O3 wMgO wK2O wTiO2 wNa2O wV2O5 wP2O5 wBaO wZnO A0 52.6 2.15 0.886 30.6 9.20 0.801 1.640 1.29 0.231 0.098 8 0.060 3 0.029 6 0.027 7 A1 40.6 13.0 1.340 23.8 15.10 1.240 1.230 1.36 0.322 0.146 0 0.298 0 0.056 8 0.032 4 A2 34.3 17.7 4.120 20.1 17.6 1.420 0.936 1.21 0.297 0.186 0 0.401 0 0.055 4 0.035 7 表 6 结渣倾向性判别结果
Table 6. The discriminant results of slagging tendency
判别指数 渣样标号 判断依据 结果 A0 A1 A2 轻微 中等 严重 $ \frac{m_{\mathrm{SiO}_2}}{m_{\mathrm{Al}_2 \mathrm{O}_3}} $ 1.72 1.71 1.71 <1.87 1.87~2.65 >2.65 不易结渣 -
[1] 孟江涛, 王菁, 杨凤玲, 等. 贫氧气氛下固硫剂对煤燃烧特性及S和N释放规律的影响[J]. 煤炭转化, 2020, 43(5): 27-37. https://www.cnki.com.cn/Article/CJFDTOTAL-MTZH202005004.htmMeng Jiangtao, Wang Jing, Yang Fengling, et al. Effects of sulfurfixing agent on coal combustion characteristics and S, N release law in condition of lean oxygen[J]. Coal Conversion, 2020, 43(5): 27-37. https://www.cnki.com.cn/Article/CJFDTOTAL-MTZH202005004.htm [2] 朱全力. 复合钙基固硫添加剂对煤固硫与燃烧的影响[J]. 环境工程学报, 2014, 8(12): 5413-5418. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201412060.htmZhu Quanli. Effect of calcium-based composite additives on combustion and sulfur retention of an anthracite coal[J]. Chinese Journal of Environmental Engineering, 2014, 8(12): 5413-5418. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201412060.htm [3] 钱剑青, 任有中, 李洪强, 等. 固硫洁净型煤的工业性应用研究[J]. 浙江大学学报: 工学版, 2001, 35(4): 356-359. doi: 10.3785/j.issn.1008-973X.2001.04.002Qian Jianqing, Ren Youzhong, Li Hongqiang, et al. Research on the industrial utilization of and combustion of clean desulfurized briquette[J]. Journal of Zhejiang University: Engineering Science, 2001, 35(4): 356-359. doi: 10.3785/j.issn.1008-973X.2001.04.002 [4] 陈列绒, 葛岭梅, 周安宁. 用钠碱改性氢氧化钙作型煤固硫剂的研究[J]. 洁净煤技术, 2005, 11(3): 73-75. doi: 10.3969/j.issn.1006-6772.2005.03.022Chen Lierong, Ge Lingmei, Zhou Anning. Study on modified calcium hydroxide with sodium alkali used as briquette capturing sulfur agent[J]. Clean Coal Technology, 2005, 11(3): 73-75. doi: 10.3969/j.issn.1006-6772.2005.03.022 [5] Cheng J, Zhou J H, Liu J Z, et al. Sulfur removal at high temperature during coal combustion in furnaces: a review[J]. Progress in Energy and Combustion Science, 2003, 29(5): 381-405. doi: 10.1016/S0360-1285(03)00030-3 [6] 张雷, 田园, 路春美. 工业废弃物固硫反应的动力学研究[J]. 中国电机工程学报, 2011, 31(2): 39-44. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGDC201102008.htmZhang Lei, Tian Yuan, Lu Chunmei. Study on the desulfurization reaction kinetics of industrial wastes[J]. Proceedings of the CSEE, 2011, 31(2): 39-44. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGDC201102008.htm [7] 翁卫国, 周俊虎, 程军, 等. 工业废渣在煤燃烧中固硫的影响因素分析[J]. 煤炭学报, 2005, 30(4): 480-483. doi: 10.3321/j.issn:0253-9993.2005.04.017Weng Weiguo, Zhou Junhu, Cheng Jun, et al. Influence factors on sulfation reactions of industrial wastes in coal combustion[J]. Journal of China Coal Society, 2005, 30(4): 480-483. doi: 10.3321/j.issn:0253-9993.2005.04.017 [8] 郑斌, 路春美, 姬丽霞, 等. 废弃物型固硫剂的固硫性能研究[J]. 中国电机工程学报, 2009, 29(11): 32-38. doi: 10.3321/j.issn:0258-8013.2009.11.006Zheng Bin, Lu Chunmei, Ji Lixia, et al. Study on the desulphurization characteristics of waste desulfurizer[J]. Proceedings of the CSEE, 2009, 29(11): 32-38. doi: 10.3321/j.issn:0258-8013.2009.11.006 [9] 刘伟, 徐东耀, 陈佐会, 等. 新型固硫剂的研发与应用研究[J]. 矿业科学学报, 2018, 3(4): 398-405. http://kykxxb.cumtb.edu.cn/article/id/165Liu Wei, Xu Dongyao, Chen Zuohui, et al. Research on the development and application of new sulfur fixing agent[J]. Journal of Mining Science and Technology, 2018, 3(4): 398-405. http://kykxxb.cumtb.edu.cn/article/id/165 [10] Yang Q W, Zhu Y, Liu C, et al. Research on the sulfur-fixing effect of compound sulfur-fixing agents from carbide slag and wastes[J]. Applied Mechanics and Materials, 2013, 316/317: 312-315. doi: 10.4028/www.scientific.net/AMM.316-317.312 [11] 何玉鑫, 华苏东, 瞿县, 等. 钢渣资源化研究进展[J]. 工业建筑, 2014, 44(S1): 948-950, 964. https://www.cnki.com.cn/Article/CJFDTOTAL-GYJZ2014S1232.htmHe Yuxin, Hua Sudong, Qu Xian, et al. Research progress of steel slag resource utilization[J]. Industr-ial Construction, 2014, 44(S1): 948-950, 964. https://www.cnki.com.cn/Article/CJFDTOTAL-GYJZ2014S1232.htm [12] 兴超, 姚娜, 张利武. SiO2含量对钢渣自粉化影响的机理研究[J]. 矿产综合利用, 2016(6): 89-91. doi: 10.3969/j.issn.1000-6532.2016.06.021Xing Chao, Yao Na, Zhang Liwu. Study on the mechanism of SiO2 content in the steel slag selfdisintegration[J]. Multipurpose Utilization of Mine-ral Resources, 2016(6): 89-91. doi: 10.3969/j.issn.1000-6532.2016.06.021 [13] 赵立杰, 张芳. 钢渣资源综合利用及发展前景展望[J]. 材料导报, 2020, 34(S2): 1319-1322, 1333. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2020S2065.htmZhao Lijie, Zhang Fang. Comprehensive utilization and development prospect of steel slag resources full text replacement[J]. Materials Reports, 2020, 34(S2): 1319-1322, 1333. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2020S2065.htm [14] 吴跃东, 彭犇, 吴龙, 等. 国内外钢渣处理与资源化利用技术发展现状综述[J]. 环境工程, 2021, 39(1): 161-165. https://www.cnki.com.cn/Article/CJFDTOTAL-HJGC202101025.htmWu Yuedong, Peng Ben, Wu Long, et al. Review on global development of treatment and utilization of steel slag[J]. Environmental Engineering, 2021, 39(1): 161-165. https://www.cnki.com.cn/Article/CJFDTOTAL-HJGC202101025.htm [15] 罗晓, 张峻搏, 何磊, 等. 钢渣对水体中磷的去除性能及机制解析[J]. 环境科学, 2021, 42(5): 2324-2333. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ202105027.htmLuo Xiao, Zhang Junbo, He Lei, et al. Analysis of the performance and mechanism of phosphorus removal in water by steel slag[J]. Environmental Science, 2021, 42(5): 2324-2333. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ202105027.htm [16] 刘泽, 李丽, 邵宁宁, 等. 钢渣-粉煤灰水热合成方沸石及其性能表征[J]. 矿业科学学报, 2018, 3(5): 508-514. http://kykxxb.cumtb.edu.cn/article/id/178Liu Ze, Li Li, Shao Ningning, et al. Hydrothermal synthesis of analcime from steel slagfly ash and its performance characterization[J]. Journal of Mining Science and Technology, 2018, 3(5): 508-514. http://kykxxb.cumtb.edu.cn/article/id/178 [17] 张朝晖, 廖杰龙, 巨建涛, 等. 钢渣处理工艺与国内外钢渣利用技术[J]. 钢铁研究学报, 2013, 25(7): 1-4. https://www.cnki.com.cn/Article/CJFDTOTAL-IRON201307002.htmZhang Zhaohui, Liao Jielong, Ju Jiantao, et al. Treatment process and utilization technology of steel slag in China and abroad[J]. Journal of Iron and Steel Research, 2013, 25(7): 1-4. https://www.cnki.com.cn/Article/CJFDTOTAL-IRON201307002.htm [18] Dhoble Y N, Ahmed S. Review on the innovative uses of steel slag for waste minimization[J]. Journal of Material Cycles and Waste Management, 2018, 20(3): 1373-1382. doi: 10.1007/s10163-018-0711-z [19] 张玉柱, 雷云波, 李俊国, 等. 钢渣矿相组成及其显微形貌分析[J]. 冶金分析, 2011, 31(9): 11-17. doi: 10.3969/j.issn.1000-7571.2011.09.003Zhang Yuzhu, Lei Yunbo, Li Junguo, et al. Analysis of mineralogical composition in steel slag and its microstructure[J]. Metallurgical Analysis, 2011, 31(9): 11-17. doi: 10.3969/j.issn.1000-7571.2011.09.003 [20] 郑瑛. 几种添加剂对石灰石固硫影响的研究[J]. 煤炭转化, 2003, 26(2): 68-71. doi: 10.3969/j.issn.1004-4248.2003.02.015Zheng Ying. Study on the influence of additives on desulphurization properties of limestone[J]. Coal Conversion, 2003, 26(2): 68-71. doi: 10.3969/j.issn.1004-4248.2003.02.015 [21] 李金洪, 鲁安怀. 钙基矿物固硫剂对不同煤种固硫效果的影响研究[J]. 环境污染治理技术与设备, 2006(11): 35-40. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200611007.htmLi Jinhong, Lu Anhuai. Effect of Ca-based mineral sulfur-fixing agent on sulfur retention of different kinds of coals[J]. Techniques and Equipment for Environmental Pollution Control, 2006(11): 35-40. . https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200611007.htm [22] 李莹英, 郭彦霞, 程芳琴, 等. 复合固硫剂对型煤固硫的影响研究[J]. 环境工程学报, 2011, 5(7): 1592-1597. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201107031.htmLi Yingying, Guo Yanxia, Cheng Fangqin, et al. Effect of compound sulfur retention agent on sulfur retention behaviors of coal briquettes[J]. Chinese Journal of Environmental Engineering, 2011, 5(7): 1592-1597. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201107031.htm [23] 陈鸿伟, 王春波, 周兰欣, 等. 复合调质提高CaO固硫率的试验研究[J]. 环境科学学报, 1999, 19(4): 357-361. doi: 10.3321/j.issn:0253-2468.1999.04.003Chen Hongwei, Wang Chunbo, Zhou Lanxin, et al. Experimental investigation on enhancing CaO desulphurization efficiency by multiply modifying[J]. Acta Scientiae Circumstantiae, 1999, 19(4): 357-361. doi: 10.3321/j.issn:0253-2468.1999.04.003 [24] 刘豪, 邱建荣, 谢峻林, 等. 铝基矿物在钙基固硫过程中的固相反应机理分析[J]. 工程热物理学报, 2007, 28(2): 351-353. doi: 10.3321/j.issn:0253-231X.2007.02.053Liu Hao, Qiu Jianrong, Xie Junlin, et al. Solid state reaction mechanism of aluminiferous mineral in the process of Ca-based sorbents desulfurization[J]. Journal of Engineering Thermophysics, 2007, 28(2): 351-353. doi: 10.3321/j.issn:0253-231X.2007.02.053 [25] 尹士吉, 程世庆, 赵斌. 高温固硫剂的试验研制[J]. 燃烧科学与技术, 1998, 4(4): 417-422. https://www.cnki.com.cn/Article/CJFDTOTAL-RSKX804.014.htmYin Shiji, Cheng Shiqing, Zhao Bin. Experimental research on desulfuriser used in high temperature[J]. Journal of Combustion Science and Technology, 1998, 4(4): 417-422. https://www.cnki.com.cn/Article/CJFDTOTAL-RSKX804.014.htm [26] 刘彦, 周俊虎, 赵晓辉, 等. Na2CO3对O2/CO2气氛下CaCO3固硫特性的影响研究[J]. 高校化学工程学报, 2005, 19(2): 263-267. doi: 10.3321/j.issn:1003-9015.2005.02.023Liu Yan, Zhou Junhu, Zhao Xiaohui, et al. The effect of Na2CO3 on direct sulfuration of CaCO3 in the atmosphere of O2/CO2[J]. Journal of Chemical Engineering of Chinese Universities, 2005, 19(2): 263-267. doi: 10.3321/j.issn:1003-9015.2005.02.023 [27] 张力, 屈紫懿, 唐强, 等. 3种添加剂对石灰石固硫效率的影响[J]. 重庆大学学报: 自然科学版, 2007, 30(8): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-FIVE200708008.htmZhang Li, Qu Ziyi, Tang Qiang, et al. Experimental study of three additives effect on limestone desulfurization efficiency[J]. Journal of Chongqing University: Natural Science Edition, 2007, 30(8): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-FIVE200708008.htm [28] Laursen K, Kern A A, Grace J R, et al. Character-ization of the enhancement effect of Na2CO3 on the sulfur capture capacity of limestones[J]. Environmental Science & Technology, 2003, 37(16): 3709-3715. [29] 郭锋, 武增华, 崔爱莉, 等. 复合钙硅固硫剂的固硫反应动力学研究[J]. 高等学校化学学报, 2003, 24(1): 100-104. doi: 10.3321/j.issn:0251-0790.2003.01.015Guo Feng, Wu Zenghua, Cui Aili, et al. Desulfurization and kinetic studies on CaCO3-SiO2 complex desulfurization reagent[J]. Chemical Research in Chinese Universities, 2003, 24(1): 100-104. doi: 10.3321/j.issn:0251-0790.2003.01.015 -





 下载:
下载: