Preparation of NaA zeolite with fly ash and its adsorption properties for lead ions
-
摘要: 以粉煤灰为原料,采用碱熔-水热法合成NaA型分子筛。通过单因素试验探究了水热温度、水热时间、碱浓度、碱灰比对制备分子筛的影响,采用静态饱和吸水量和钙离子交换量对所制备分子筛的性能进行评价。结果表明:水热温度100 ℃、水热时间5 h、NaOH浓度2.73 mol/L、碱灰比2.8有利于NaA型分子筛的合成,其钙离子交换量最高可达374.63 mg/g。利用合成的NaA型分子筛对液相中的铅离子进行吸附研究,在分子筛投加量1 g/L、溶液pH值6.2、吸附温度25 ℃、吸附60 min时,吸附容量(Qe)最高可达471.51 mg/g;分子筛的吸附等温线更加符合Langmuir等温线模型,饱和吸附容量(Qm)可达580.18 mg/g。Abstract: This study synthesized NaA zeolite by alkali melting-hydrothermal method based on fly ash as raw material. The effects of hydrothermal temperature, hydrothermal time, alkali concentration and alkali/FA mass ratio on the preparation of zeolites were investigated by single factor experiments, and the performance of the prepared zeolites was evaluated by water adsorption capacity and Cation Exchange Capacity (CEC) value.Results show that it is beneficial to the synthesis of NaA zeolite under hydrothermal temperature of 100 ℃, hydrothermal time of 5 h, NaOH concentration of 2.73 mol/L, and alkali/FA mass ratio of 2.8, in which the highest CEC value can reach 374.63 mg/g. The adsorption of Pb(Ⅱ) in liquid phase was studied by using the synthetic NaA zeolite. The adsorption capacity was up to 471.51 mg/g with 1 g/L dosage of zeolite, at pH 6.2 and 25 ℃ of adsorption temperature and the adsorption time was 60 min.The adsorption data of Pb(Ⅱ) by NaA zeolite was fitted better with Langmuir model, and the saturated adsorption capacity of Pb(Ⅱ) could reach to 580.18 mg/g.
-
Key words:
- fly ash /
- NaA zeolite /
- alkali melting-hydrothermal /
- lead ion /
- adsorption
-
表 1 粉煤灰化学成分
Table 1. Chemical compositions of FA
% 化学组成 SiO2 Al2O3 Fe2O3 CaO SO3 TiO2 MgO K2O Na2O Cl P2O5 质量分数 51.18 36.76 3.21 2.74 1.78 1.35 1.16 0.98 0.25 0.15 0.20 表 2 动力学拟合参数
Table 2. Kinetic fitting parameters for Pb(Ⅱ) adsorption
模型 Qe/(mg·g-1) k R2 准一级动力学模型 453.09 0.295 6 0.989 8 准二级动力学模型 477.18 0.001 3 0.999 3 表 3 吸附等温线拟合参数
Table 3. Langmuir and Freundlich parameters for Pb(Ⅱ) adsorption
Langmuir参数 Freundlich参数 b/(L·mg-1) Qm/(mg·g-1) R2 k n R2 0.143 1 580.18 0.991 0 375.16 13.43 0.909 7 表 4 各类分子筛的Pb(Ⅱ)吸附容量
Table 4. Pb(Ⅱ) adsorption capacity of all kinds of molecular sieves
-
[1] Temuujin J, Surenjav E, Ruescher C H, et al. Processing and uses of fly ash addressing radioactivity (critical review)[J]. Chemosphere, 2019, 216: 866-882. doi: 10.1016/j.chemosphere.2018.10.112 [2] Panda L, Dash S. Characterization and utilization of coal fly ash: a review[J]. Emerging Materials Research, 2020, 9(3): 921-934. doi: 10.1680/jemmr.18.00097 [3] 蒋斌斌, 高昊, 杜坤, 等. 煤基复合絮凝剂对高浊度高矿化度矿井水的絮凝效果研究[J]. 矿业科学学报, 2020, 5(6): 681-686. doi: 10.19606/j.cnki.jmst.2020.06.011Jiang Binbin, Gao Hao, Du Kun, et al. Research on flocculation effect of fly ash composite on high turbidity and high salinity mine water[J]. Journal of Mining Science and Technology, 2020, 5(6): 681-686. doi: 10.19606/j.cnki.jmst.2020.06.011 [4] 杨跃翔, 王锟桠, 任蕾. 中国煤炭工业高质量发展测度及耦合协调分析: 基于2000—2019年数据的实证研究[J]. 矿业科学学报, 2021, 6(6): 764-776. doi: 10.19606/j.cnki.jmst.2021.06.016Yang Yuexiang, Wang Kunya, Ren Lei. Evaluation and coupling coordination analysis of high quality development of China's coal industry: empirical research based on data from 2000 to 2019[J]. Journal of Mining Science and Technology, 2021, 6(6): 764-776. doi: 10.19606/j.cnki.jmst.2021.06.016 [5] 姜龙. 燃煤电厂粉煤灰综合利用现状及发展建议[J]. 洁净煤技术, 2020, 26(4): 31-39. https://www.cnki.com.cn/Article/CJFDTOTAL-JJMS202004004.htmJiang Long. Comprehensiveutilization situation of fly ash in coal-fired power plants and its development suggestions[J]. Clean Coal Technology, 2020, 26(4): 31-39. https://www.cnki.com.cn/Article/CJFDTOTAL-JJMS202004004.htm [6] 汤倩. 粉煤灰利用研究现状及其在环境保护中的应用[J]. 中国资源综合利用, 2020, 38(5): 41-43. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWZS202005014.htmTang Qian. Research status of fly ash utilization and its application in environmental protection[J]. China Resources Comprehensive Utilization, 2020, 38(5): 41-43. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWZS202005014.htm [7] Jiang C L, Wang R, Chen X, et al. Preparation of chitosan modified fly ash under acid condition and its adsorption mechanism for Cr(Ⅵ) in water[J]. Journal of Central South University, 2021, 28(6): 1652-1664. doi: 10.1007/s11771-021-4724-8 [8] Zhang J J, Zhang X Y, Liu B, et al. Phase evolution and properties of glass ceramic foams prepared by bottom ash, fly ash and pickling sludge[J]. International Journal of Minerals, Metallurgy and Materials, 2022, 29(3): 563-573. doi: 10.1007/s12613-020-2219-5 [9] Zong Y B, Zhao C Y, Chen W H, et al. Preparation of hydro-sodalite from fly ash using a hydrothermal method with a submolten salt system and study of the phase transition process[J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(1): 55-62. doi: 10.1007/s12613-019-1904-8 [10] 孙延文, 王连勇, 杨湘澜, 等. 粉煤灰基沸石的制备及应用研究进展[J]. 硅酸盐通报, 2021, 40(1): 123-132. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202101016.htmSun Yanwen, Wang Lianyong, Yang Xianglan, et al. Research progress on synthesis and application of zeolite based on coal fly ash[J]. Bulletin of the Chinese Ceramic Society, 2021, 40(1): 123-132. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202101016.htm [11] Zhang X Y, Li C Q, Zheng S L, et al. A review of the synthesis and application of zeolites from coal-based solid wastes[J]. International Journal of Minerals, Metallurgy and Materials, 2022, 29(1): 1-21. doi: 10.1007/s12613-021-2256-8 [12] 于成龙, 熊楠, 宋杰, 等. 近20年来中国利用粉煤灰合成分子筛研究进展[J]. 矿产综合利用, 2020(4): 26-35. https://www.cnki.com.cn/Article/CJFDTOTAL-KCZL202004005.htmYu Chenglong, Xiong Nan, Song Jie, et al. Development of molecular sieves composition from fly ash in China in the last two decades[J]. Multipurpose Utilization of Mineral Resources, 2020(4): 26-35. https://www.cnki.com.cn/Article/CJFDTOTAL-KCZL202004005.htm [13] Lee Y R, Soe J T, Zhang S Q, et al. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: a mini review[J]. Chemical Engineering Journal, 2017, 317: 821-843. doi: 10.1016/j.cej.2017.02.124 [14] 刘玉慧, 陈天虎, 王灿, 等. 风化花岗岩洗砂尾泥制备4A沸石高效氨氮吸附剂[J]. 硅酸盐学报, 2021, 49(7): 1429-1438. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB202107019.htmLiu Yuhui, Chen Tianhu, Wang Can, et al. 4A zeolite derived from weathered washing sand tail mud as A high-efficiency NH4+-N adsorbent[J]. Journal of the Chinese Ceramic Society, 2021, 49(7): 1429-1438. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB202107019.htm [15] 白彪坤, 孟岳, 陈叔平. 4A分子筛的吸附性能与孔结构研究[J]. 硅酸盐通报, 2020, 39(10): 3367-3372. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202010046.htmBai Biaokun, Meng Yue, Chen Shuping. Adsorption performance and pore structure of 4A molecular sieve[J]. Bulletin of the Chinese Ceramic Society, 2020, 39(10): 3367-3372. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202010046.htm [16] 李建, 石应杰, 付晓恒, 等. 粉煤灰基介孔ZSM-5的孔径调控以及催化油烟中戊醛[J]. 中南大学学报: 自然科学版, 2020, 51(5): 1174-1188. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD202005002.htmLi Jian, Shi Yingjie, Fu Xiaoheng, et al. Aperture adjustment of mesoporous ZSM-5 based on fly ash for catalysis of pentanal in cooking fumes[J]. Journal of Central South University: Science and Technology, 2020, 51(5): 1174-1188. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD202005002.htm [17] 杨建利, 杜美利, 郭明, 等. 澄合粉煤灰制备NaY分子筛及其离子交换吸附性能研究[J]. 离子交换与吸附, 2015, 31(4): 317-324. https://www.cnki.com.cn/Article/CJFDTOTAL-LJYX201504004.htmYang Jianli, Du Meili, Guo Ming, et al. Preparation and adsorption performance of NaY zeolite from fly ash in Chenghe[J]. Ion Exchange and Adsorption, 2015, 31(4): 317-324. https://www.cnki.com.cn/Article/CJFDTOTAL-LJYX201504004.htm [18] 周立峰, 任栋, 谷悦, 等. 粉煤灰基磁性A型沸石的合成及吸附性能研究[J]. 冶金能源, 2018, 37(6): 54-58. https://www.cnki.com.cn/Article/CJFDTOTAL-YJLY201806014.htmZhou Lifeng, Ren Dong, Gu Yue, et al. Synthesis and adsorption properties of fly ash-based magnetic type A zeolite[J]. Energy for Metallurgical Industry, 2018, 37(6): 54-58. https://www.cnki.com.cn/Article/CJFDTOTAL-YJLY201806014.htm [19] Zhang B P, Chen Y L, Wei L, et al. Preparation of molecular sieve X from coal fly ash for the adsorption of volatile organic compounds[J]. Microporous and Mesoporous Materials, 2012, 156: 36-39. doi: 10.1016/j.micromeso.2012.02.016 [20] 贺龙强, 胡鹏, 付克明. 利用粉煤灰制备分子筛及对水体中六价铬的吸附研究[J]. 硅酸盐通报, 2017, 36(10): 3493-3497, 3503. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201710045.htmHe Longqiang, Hu Peng, Fu Keming. Adsorption of hexavalent chromium by zeolite synthesized of fly ash[J]. Bulletin of the Chinese Ceramic Society, 2017, 36(10): 3493-3497, 3503. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201710045.htm [21] 李晓光, 赵颖, 康得军, 等. 粉煤灰基X型沸石分子筛的合成制备及其去除氨性能研究[J]. 硅酸盐通报, 2018, 37(11): 3663-3668. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201811050.htmLi Xiaoguang, Zhao Ying, Kang Dejun, et al. Synthesis of pure X zeolite sieve from coal fly ash for ammonium removal[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(11): 3663-3668. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201811050.htm [22] Tosheva L, Valtchev V P. Nanozeolites: synthesis, crystallization mechanism, and applications[J]. ChemInform, 2005, 36(29): 2494-2513. [23] Murayama N, Yamamoto H, Shibata J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction[J]. International Journal of Mineral Processing, 2002, 64(1): 1-17. doi: 10.1016/S0301-7516(01)00046-1 [24] 张徐宁. 粉煤灰合成沸石分子筛及其对铅离子的吸附性能研究[D]. 太原: 太原理工大学, 2012. [25] 石飞, 刘红, 刘鲁建, 等. 4A分子筛对水中铅离子的吸附及其机理分析[J]. 环境科学与技术, 2014, 37(12): 154-159, 173. https://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201412032.htmShi Fei, Liu Hong, Liu Lujian, et al. Adsorption of lead ions from aqueous solution onto 4A molecular sieve and mechanism analysis[J]. Environmental Science & Technology, 2014, 37(12): 154-159, 173. https://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201412032.htm [26] 孙秀云, 马芳变, 施筱堃, 等. 粉煤灰合成介孔分子筛SBA-15对Pb(Ⅱ)离子的吸附[J]. 中南大学学报: 自然科学版, 2014, 45(11): 4093-4099. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201411052.htmSun Xiuyun, Ma Fangbian, Shi Xiaokun, et al. Adsorption of Pb(Ⅱ) on SBA-15 synthesized from fly ash[J]. Journal of Central South University: Science and Technology, 2014, 45(11): 4093-4099. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNGD201411052.htm [27] 贺框. 粉煤灰制备NaA型沸石分子筛及其对重金属离子吸附的研究[D]. 广州: 华南理工大学, 2016. [28] 李超, 王丽萍, 郭昭华, 等. 粉煤灰提铝后尾渣合成13X分子筛及其对Pb2+吸附性能的研究[J]. 矿产保护与利用, 2018(6): 98-102. https://www.cnki.com.cn/Article/CJFDTOTAL-KCBH201806021.htmLi Chao, Wang Liping, Guo Zhaohua, et al. Synthesis of 13X zeolite by fly ash acid residue and its adsorption performance on lead ions[J]. Conservation and Utilization of Mineral Resources, 2018(6): 98-102. https://www.cnki.com.cn/Article/CJFDTOTAL-KCBH201806021.htm [29] Panek R, Medykowska M, Szewczuk-Karpisz K, et al. Comparison of physicochemical properties of fly ash precursor, Na-P1(C) zeolite-carbon composite and Na-P1 zeolite-adsorption affinity to divalent Pb and Zn cations[J]. Materials: Basel, Switzerland, 2021, 14(11): 3018. [30] Liu H B, Peng S C, Shu L, et al. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+[J]. Chemosphere, 2013, 91(11): 1539-1546. -
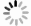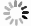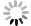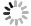





 下载:
下载: