Study on hydration mechanism of calcium silicon slag composite geopolymer
-
摘要: 为实现硅钙渣、粉煤灰及矿渣三种固废的协同利用,本文通过开展不同粉煤灰、矿渣比(灰渣比)下的硅钙渣复合地聚物制备实验,对硅钙渣复合地聚物的水化机理进行了研究。结果表明,硅钙渣复合地聚物是由β-硅酸二钙自身水化和碱激发水化共同形成的一种以C—S—H和C(N)—A—S—H为主的二元复合胶凝材料;相较于晶相矿物,玻璃相矿物更易发生碱激发水化反应,导致灰渣比在0.5以上时7 d水化物中残存大量未反应的莫来石,但随养护时间的延长莫来石会继续进行水化,并在28 d时生成蠕虫状四方钠沸石和条状贝德石。同时在灰渣比为1.0时,硅钙渣地聚物微观形貌最均匀致密,28 d抗压强度最高,达到37.9 MPa,说明此时能够发挥出粉煤灰、矿渣、硅钙渣之间最佳的协同效应。Abstract: In order to realize the synergistic utilization of calcium silicon slag, fly ash and blast-furnace slag, this paper researched on hydration mechanism of calcium silicon slag composite geopolymer through preparation experiments of the calcium silicon slag composite geopolymer under different ratio of fly ash / blast-furnace slag.The results show that the calcium silicate slag base geopolymer is a binary composite cementitious material mainly composed of C—S—H and C(N)—A—S—H, which is formed by the hydration of β-calcium silicate itself and alkali-activated hydration.Compared with crystalline minerals, glass minerals are more prone to reaction with Ca(OH)2 and sodium silicate, resulting in a large amount of unreacted mullite remaining in the hydrates of 7 d when the fly ash / blast-furnace slag ratio is more than 0.5, but the mullite will continue to hydrate with the extension of curing time, and worm-like tetranatrolite and strip-like beidellite will be formed at 28 d.At the same time, when fly ash / blast-furnace slag ratio is 1.0, micromorphology of calcium silicate slag composite geopolymer is the most evenly distributed and dense, and its 28 d compressive strength reaches maximum 37.9 MPa.So, it indicates that the best synergistic effect among calcium silicon slag, fly ash and blast-furnace slag can be released under this condition.
-
Key words:
- calcium silicon slag /
- geopolymer /
- fly ash /
- blast-furnace slag /
- hydrates
-
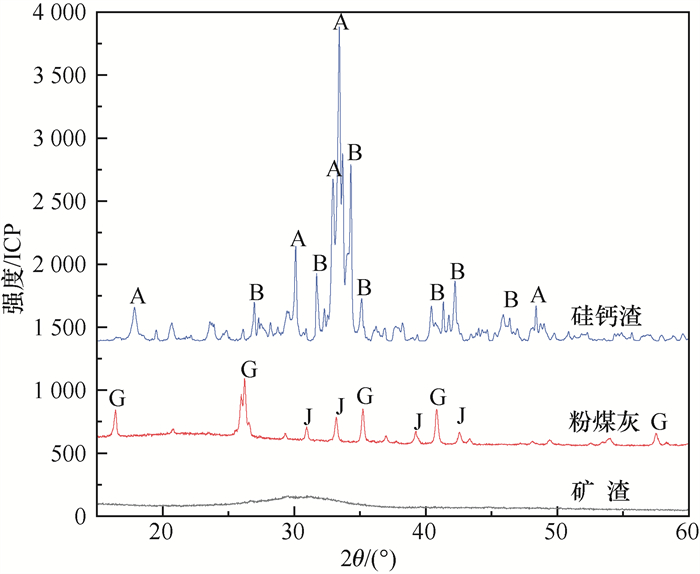
图 2 硅钙渣复合地聚物XRD分析
A—β-硅酸二钙(β-2CaO·SiO2);B—方解石(CaCO3);C—二水钙长石(CaO·Al2O3·2SiO2·2H2O);D—水化硅酸钙(CaO·SiO2·H2O);E—钠钙沸石(Na3.7Ca7.4Al18.5Si77.5O192·74H2O);F—托勃莫来石(5CaO·6SiO2·5H2O);G—莫来石(3Al2O3·2SiO2);H—四方钠沸石[(Na,Ca)2(Si,Al)5O10·2H2O];I—贝德石[Na0.3Al2(Si,Al)4O10(OH)2·2H2O]
Figure 2. XRD patterns of calcium silicon slag composite geopolymer
表 1 原料化学成分(质量分数)
Table 1. The chemical constituents of raw materials
% 成分 SiO2 Fe2O3 Al2O3 CaO MgO Na2O K2O SO3 P2O5 F Cl 硅钙渣 31.08 2.25 5.97 50.35 3.61 2.31 0.36 3.21 0.42 0.15 0.29 矿渣 34.57 0.51 10.50 42.75 4.13 0.77 0.46 2.78 3.34 0.12 0.07 粉煤灰 42.67 2.57 42.36 4.30 3.20 0.58 0.39 1.27 1.46 0.47 0.73 波数/cm-1 对应基团振动 3 450~3 000 水分子伸缩振动 1 650~1 600 水分子弯曲振动 1 450~1 400 CO32-的非对称伸缩振动 975~965 C—S—H非对称伸缩振动 950~900 —Si—O—Si(Al)—非对称伸缩振动 890~850 —Si(Al)—OH弯曲振动 730~710 —Si—O—Si(Al)—弯曲振动 表 3 硅钙渣复合地聚物水化物EDS能谱及对应的物相
Table 3. EDS analysis of hydrates for calcium silicon slag composite geopolymer and corresponding phase
% 区域 形貌 各元素摩尔分数比 物相 O Al Si Ca Na Mg Fe C 1 球状 55.23 2.31 14.22 26.50 0.21 1.22 0.31 — β-硅酸二钙 2 片状 63.71 0.52 17.22 16.72 — 0.81 1.02 — 水化硅酸钙 3 鳞片状 59.22 0.22 0.10 20.03 — 0.10 0.10 20.23 方解石 4 絮状 70.21 5.03 20.64 2.02 2.10 — — — 钠钙沸石 5 棒状 65.36 0.32 18.25 15.13 — 0.52 0.42 — 托勃莫来石 6 棒状 66.03 0.26 18.45 14.83 — 0.22 0.21 — 托勃莫来石 7 块状 61.46 27.71 9.52 — 0.27 — 1.04 — 莫来石 8 块状 60.24 26.98 10.96 — 0.55 0.45 0.82 — 莫来石 9 块状 62.12 26.56 10.26 — 0.23 0.12 0.71 — 莫来石 10 球状 54.56 1.75 15.42 26.22 0.13 1.52 0.40 — β-硅酸二钙 11 片状 65.22 0.33 17.45 16.22 0.13 0.44 0.21 — 水化硅酸钙 12 蠕虫状 63.23 5.21 21.01 5.23 5.01 0.11 0.20 — 四方钠沸石 13 蠕虫状 62.98 5.67 19.82 5.56 5.44 0.32 0.21 — 四方钠沸石 14 蠕虫状 62.65 5.27 21.05 5.16 5.31 0.22 0.34 — 四方钠沸石 15 条状 68.97 14.63 14.36 0.22 1.61 0.11 0.10 — 贝德石 16 条状 69.29 14.19 14.38 0.11 1.82 0.12 0.09 — 贝德石 17 条状 68.62 14.61 14.45 0.19 2.03 — 0.10 — 贝德石 -
[1] Wang P, Wang J M, Qin Q, et al. Life cycle assessment of magnetized fly-ash compound fertilizer production: a case study in China[J]. Renewable and Sustainable Energy Reviews, 2017, 73: 706-713. doi: 10.1016/j.rser.2017.02.005 [2] Yang C N, Zhang J B, Li S P, et al. Mechanisms of mechanochemical activation during comprehensive utilization of high-alumina coal fly ash[J]. Waste Management, 2020, 116: 190-195. doi: 10.1016/j.wasman.2020.08.003 [3] Shi Y, Jiang K X, Zhang T A. A cleaner electrolysis process to recover alumina from synthetic sulfuric acid leachate of coal fly ash[J]. Hydrometallurgy, 2020, 191: 105196. doi: 10.1016/j.hydromet.2019.105196 [4] Wang L, Zhang T A, Lü G Z, et al. Carbochlorination of alumina and silica from high-alumina fly ash[J]. Minerals Engineering, 2019, 130: 85-91. doi: 10.1016/j.mineng.2018.09.022 [5] Rui H M, Zhang L C, Li L J, et al. Solvent extraction of lithium from hydrochloric acid leaching solution of high-alumina coal fly ash[J]. Chemical Physics Letters, 2021, 771: 138510. doi: 10.1016/j.cplett.2021.138510 [6] Sun Y L, Qi G X, Lei X F, et al. Extraction of uranium in bottom ash derived from high-germanium coals[J]. Procedia Environmental Sciences, 2016, 31: 589-597. doi: 10.1016/j.proenv.2016.02.096 [7] Hu P P, Hou X J, Zhang J B, et al. Distribution and occurrence of lithium in high-alumina-coal fly ash[J]. International Journal of Coal Geology, 2018, 189: 27-34. doi: 10.1016/j.coal.2018.02.011 [8] 李会泉, 张建波, 王晨晔, 等. 高铝粉煤灰伴生资源清洁循环利用技术的构建与研究进展[J]. 洁净煤技术, 2018, 24(2): 1-8. https://www.cnki.com.cn/Article/CJFDTOTAL-JJMS201802001.htmLi Huiquan, Zhang Jianbo, Wang Chenye, et al. Construct and research advance in clean and cyclic utilizations of associated resources in high-alumina coal fly ash[J]. Clean Coal Technology, 2018, 24(2): 1-8. https://www.cnki.com.cn/Article/CJFDTOTAL-JJMS201802001.htm [9] Zhang J B, Li S P, Li H Q, et al. Acid activation for pre-desilicated high-alumina fly ash[J]. Fuel Processing Technology, 2016, 151: 64-71. doi: 10.1016/j.fuproc.2016.05.036 [10] 贺实月, 李会泉, 李少鹏, 等. 煤粉炉高铝粉煤灰碱溶脱硅反应动力学[J]. 中国有色金属学报, 2014, 24(7): 1888-1894. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201407029.htmHe Shiyue, Li Huiquan, Li Shaopeng, et al. Kinetics of desilication process of fly ash with high aluminum from pulverized coal fired boiler in alkali solution[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1888-1894. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201407029.htm [11] Yan C W, Zhao H W, Zhang J, et al. The cementitious composites using calcium silicate slag as partial cement[J]. Journal of Cleaner Production, 2020, 256: 120514. doi: 10.1016/j.jclepro.2020.120514 [12] Yang Z J, Kang D, de Zhang, et al. Crystal transformation of calcium silicate minerals synthesized by calcium silicate slag and silica fume with increase of C/S molar ratio[J]. Journal of Materials Research and Technology, 2021, 15: 4185-4192. doi: 10.1016/j.jmrt.2021.10.047 [13] Yang Z J, Zhang D, Jiao Y, et al. Crystal evolution of calcium silicate minerals synthesized by calcium silicon slag and silica fume with increase of hydrothermal synthesis temperature[J]. Materials, 2022, 15(4): 1620. doi: 10.3390/ma15041620 [14] Zhang D, Yang Z J, Kang D, et al. Experimental study on subgrade material of calcium silicate slag[J]. Materials, 2022, 15(6): 2304. doi: 10.3390/ma15062304 [15] 王晓丽, 李秋义, 陈帅超, 等. 工业固体废弃物在新型建材领域中的应用研究与展望[J]. 硅酸盐通报, 2019, 38(11): 3456-3464. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201911013.htmWang Xiaoli, Li Qiuyi, Chen Shuaichao, et al. Application research and prospect of industrial solid waste in new building materials[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(11): 3456-3464. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201911013.htm [16] 孙坚, 耿春雷, 张作泰, 等. 工业固体废弃物资源综合利用技术现状[J]. 材料导报, 2012, 26(11): 105-109. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201211023.htmSun Jian, Geng Chunlei, Zhang Zuotai, et al. Present situation of comprehensive utilization technology of industrial solid waste[J]. Materials Review, 2012, 26(11): 105-109. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201211023.htm [17] Bensted J, Barnes P. Structure and performance of cements, 2nd edition[M]. London: Applied Science Publishers, 1983. [18] Cong P L, Mei L N. Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum[J]. Construction and Building Materials, 2021, 275: 122171. doi: 10.1016/j.conbuildmat.2020.122171 [19] Nocuò-Wczelik W. Effect of Na and Al on the phase composition and morphology of autoclaved calcium silicate hydrates[J]. Cement and Concrete Research, 1999, 29(11): 1759-1767. doi: 10.1016/S0008-8846(99)00166-0 [20] 黄丽萍, 马倩敏, 郭荣鑫, 等. 碱矿渣胶凝材料水化产物的试验研究[J]. 硅酸盐通报, 2020, 39(4): 1194-1200. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202004026.htmHuang Liping, Ma Qianmin, Guo Rongxin, et al. Experimental study on hydration products of alkali-activated slag[J]. Bulletin of the Chinese Ceramic Society, 2020, 39(4): 1194-1200. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT202004026.htm [21] Wang Y G, Liu X M, Zhang W, et al. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer[J]. Journal of Cleaner Production, 2020, 244: 118852. doi: 10.1016/j.jclepro.2019.118852 [22] Criado M, Palomo A, Fernández-Jiménez A. Alkali activation of fly ashes. Part 1: effect of curing conditions on the carbonation of the reaction products[J]. Fuel, 2005, 84(16): 2048-2054. doi: 10.1016/j.fuel.2005.03.030 [23] Shi D, Yao Y, Ye J Y, et al. Effects of seawater on mechanical properties, mineralogy and microstructure of calcium silicate slag-based alkali-activated materials[J]. Construction and Building Materials, 2019, 212: 569-577. doi: 10.1016/j.conbuildmat.2019.03.288 -





 下载:
下载: