Study on operation condition of an in-situ carbon capture gasification based on transport reactor
-
摘要: 针对固定床或鼓泡流化床反应器的内在碳捕集气化过程中气固混合程度差的问题,本文将输运床作为内在碳捕集气化制氢反应器,建立了考虑流体动力学及反应动力学的反应器动力学模型,研究了水碳比、操作温度及操作压力对反应器运行可行性及性能的影响。结果表明,以输运床小试装置为基准,当反应器操作压力为900 kPa、反应器温度为800 ℃、进口水碳比为2时,反应器内碳转化率可达60 %,出口气体产物(干气)中氢气的摩尔分数可达到82 %。研究结果证实了基于输运床的内在碳捕集气化反应器的可行性及其在提高反应性能上的优势,指出了操作条件范围,可为内在碳捕集气化制氢技术的研究提供参考。Abstract: In order to solve the problem of low carbon conversion rate in the conventional in-situ carbon capture gasification process based on fixed bed or bubbling fluidized bed reactor, in this paper, a transport bed is applied as the reactor of the in-situ carbon capture gasification for hydrogen production.The dynamic model of the reactor combining hydrodynamic models and reaction kinetic models is built to investigate the operation feasibility and influence of key parameters, such as steam to carbon ratio, operation temperature and pressure, on reactor performance.The study concludes the operational conditions of the reactor when it has operational feasibility.Meanwhile, results show that, take a small test transport equipment as basis, the reactor is recommend to operate at pressure of 900 kPa, temperature of 800 ℃ and steam to carbon ratio of 2.Under this condition, the H2 composition in the dry gas could reach 82 % together with the carbon conversion of 60 %.The results confirmed the feasibility of the transport bed as the in-situ carbon capture gasification reactor and its advantage in improving the in-situ carbon capture gasification performance, determined the ranges of reactor operation conditions, which provide a reference for the development of hydrogen production technology by in-situ carbon capture gasification.
-
表 1 输运床气化模拟结果与文献的对比
Table 1. Results comparison of transport gasifier model with reference
输入参数 进口煤流量/(kg·h-1) 125.5 进口煤温度/K 295 操作压力/kPa 927 操作温度/K 1406 循环流量/(kg·h-1) 1923 气速/(m·s-1) 7.86 空气温度/K 422 空气流量/(m3·h-1) 275 吹扫气流量/(m3·h-1) 124.6 蒸汽流量/(m3·h-1) 48.4 蒸汽温度/K 573 固体吸收剂流量/(kg·h-1) 6.6 固体吸收剂温度/K 295 结果比较/% 气体成分 文献结果 模型计算结果 CO 7.8 7.73 H2 8.4 8.21 CO2 11.5 11.90 CH4 1.7 2.83 N2 71.2 69.33 碳转化率 88.5 88.47 表 2 输运床气化结果与小试结果的比较
Table 2. Results comparison of transport gasifier model with a small test
输入参数 结果比较% 给煤量/(kg·h-1) 136.96 气体成分 实验结果 模型计算结果 给柴油量/(L·h-1) 2.42 CO 13.38 11.64 空气量/(Nm3·h-1) 261.856 CO2 14.74 13.72 氧气量/(Nm3·h-1) 17.21 CH4 2.15 2.81 氮气量/(Nm3·h-1) 55.06 H2 7.13 8.23 提升管出口压力/kPa 609 N2 62.6 63.6 提升管平均温度/℃ 832 碳转化率 - 71.2 表 3 神木煤煤质分析
Table 3. The proximate and ultimate analysis of Shenmu coal
工业分析 元素分析 Mar/% Mad/% Aad/% Vad/% FCad/% DT/℃ Cad/% Had/% Oad/% Nad/% Sad/% Qnet,ar/(MJ·kg-1) 12.8 5.76 7.65 34.56 52.03 1 120 70.36 4.35 10.54 0.91 0.43 25.02 表 4 模拟过程基准参数设置
Table 4. Basic parameter settings of modelling
输入参数 气化反应器温度/℃ 气化反应器压力/kPa 气化反应器Gs/(kg·m-2s-1) 反应器给煤量/(kg·h-1) 钙碳摩尔比 水碳摩尔比 水蒸气进口温度/℃ CaO进口温度/℃ 固体颗粒平均直径/μm 颗粒球形度 煤焦及灰密度/(kg·m-3) CaO密度/(kg·m-3) CaCO3密度/(kg·m-3) 提升管高度/m 提升管内径/m 数值 750 900 500 150 1 2 550 980 154 0.7 1 450 3 300 2 800 18.5 0.1 -
[1] Dawood F, Anda M, Shafiullah G M.Hydrogen production for energy: An overview[J]. International Journal of Hydrogen Energy, 2020, 45(7): 3847-3869. doi: 10.1016/j.ijhydene.2019.12.059 [2] Abe J O, Popoola A P I, Ajenifuja E, et al. Hydrogen energy, economy and storage: Review and recommendation[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15072-15086. doi: 10.1016/j.ijhydene.2019.04.068 [3] 魏世杰, 樊静丽, 杨康迪, 等. 2011年和2016年山西省煤炭流动及碳排放对比分析[J]. 矿业科学学报, 2020, 5(3): 334-341. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract297.shtmlWei Shijie, Fan Jingli, Yang Kangdi, et al. A comparison of coal flow and carbon emissions in Shanxi province between 2011 and 2016[J]. Journal of Mining Science and Technology, 2020, 5(3): 334-341. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract297.shtml [4] 赵明东, 董东林, 田康. 煤炭地下气化覆岩温度场和裂隙场变化机制模拟研究[J]. 矿业科学学报, 2017, 2(1): 1-6. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract41.shtmlZhao Mingdong, Dong Donglin, Tian Kang. Change mechanism simulation study of the overlying strata temperature field and fracture field in UCG[J]. Journal of Mining Science and Technology, 2017, 2(1): 1-6. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract41.shtml [5] 杨洋, 祁娇, 张冉. 基于污染治理视角的洁净煤燃烧技术的经济效益和环境效益分析[J]. 矿业科学学报, 2016, 1(3): 291-298. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract40.shtmlYang Yang, Qi Jiao, Zhang Ran. Analysis of economic and environmental benefits of clean coal technology in the perspective of the pollution control[J]. Journal of Mining Science and Technology, 2016, 1(3): 291-298. http://kykxxb.cumtb.edu.cn/CN/abstract/abstract40.shtml [6] Seyitoglu S S, Dincer I, Kilicarslan A.Energy and exergy analyses of hydrogen production by coal gasification[J]. International Journal of Hydrogen Energy, 2017, 42(4): 2592-2600. doi: 10.1016/j.ijhydene.2016.08.228 [7] Zhou X, Yang X, Li J, et al. Pressurized catalytic calcium looping hydrogen generation from coal with in-situ CO2 capture[J]. Energy Conversion and Management, 2019, 198: 111899. doi: 10.1016/j.enconman.2019.111899 [8] Lin S, Harada M, Suzuki Y, et al. Process analysis for hydrogen production by reaction integrated novel gasification(HyPr-RING)[J]. Energy Conversion and Management, 2005, 46(6): 869-880. doi: 10.1016/j.enconman.2004.06.008 [9] Sinha S N, Kulkarni P K, Shah S H, et al. Environmental monitoring of benzene and toluene produced in indoor air due to combustion of solid biomass fuels[J]. Science of The Total Environment, 2006, 357(1-3): 280-287. doi: 10.1016/j.scitotenv.2005.08.011 [10] 乔春珍, 肖云汉. 碳制氢过程的比较及直接制氢分析[J]. 工程热物理学报, 2005, 26(5): 729-732. doi: 10.3321/j.issn:0253-231X.2005.05.003Qiao Chunzheng, Xiao Yunhan. Comparison of processes of hydrogen from carbon and analysis of hydrogen from carbon direct[J]. Journal of Engineering Thermophysics, 2005, 26(5): 729-732. doi: 10.3321/j.issn:0253-231X.2005.05.003 [11] Chen S, Wang D, Xue Z, et al. Calcium looping gasification for high-concentration hydrogen production with CO2 capture in a novel compact fluidized bed: Simulation and operation requirements[J]. International Journal of Hydrogen Energy, 2011, 36(8): 4887-4899. doi: 10.1016/j.ijhydene.2010.12.130 [12] Wang Q, Rong N, Fan H, et al. Enhanced hydrogen-rich gas production from steam gasification of coal in a pressurized fluidized bed with CaO as a CO2 sorbent[J]. International Journal of Hydrogen Energy, 2014, 39(11): 5781-5792. doi: 10.1016/j.ijhydene.2014.01.153 [13] Esmaili E, Mahinpey N, Lim C J.Modified equilibrium modelling of coal gasification with in situ CO2 capture using sorbent CaO: Assessment of approach temperature[J]. Chemical Engineering Research and Design, 2013, 91(7): 1361-1369. doi: 10.1016/j.cherd.2013.02.015 [14] Pitkäoja A, Ritvanen J, Hafner S, et al. Simulation of a sorbent enhanced gasification pilot reactor and validation of reactor model[J]. Energy Conversion and Management, 2020, 204: 112318. doi: 10.1016/j.enconman.2019.112318 [15] Sreejith C C, Muraleedharan C, Arun P.Air-steam gasification of biomass in fluidized bed with CO2 absorption: A kinetic model for performance prediction[J]. Fuel Processing Technology, 2015, 130: 197-207. doi: 10.1016/j.fuproc.2014.09.040 [16] Zhou L, Duan L, Anthony E J.A calcium looping process for simultaneous CO2 capture and peak shaving in a coal-fired power plant[J]. Applied Energy, 2019, 235: 480-486. doi: 10.1016/j.apenergy.2018.10.138 [17] Rolfe A, Huang Y, Haaf M, et al. Integration of the calcium carbonate looping process into an existing pulverized coal-fired power plant for CO2 capture: Techno-economic and environmental evaluation[J]. Applied Energy, 2018, 222: 169-179. doi: 10.1016/j.apenergy.2018.03.160 [18] Hu Y, Ahn H.Process integration of a Calcium-looping process with a natural gas combined cycle power plant for CO2 capture and its improvement by exhaust gas recirculation[J]. Applied Energy, 2017, 187: 480-488. doi: 10.1016/j.apenergy.2016.11.014 [19] Sun Z, Chen S, Ma S, et al. Simulation of the calcium looping process(CLP)for hydrogen, carbon monoxide and acetylene poly-generation with CO2 capture and COS reduction[J]. Applied Energy, 2016, 169: 642-651. doi: 10.1016/j.apenergy.2016.02.077 [20] Hanak D P, Manovic V.Calcium looping with supercritical CO2 cycle for decarbonisation of coal-fired power plant[J]. Energy, 2016, 102: 343-353. doi: 10.1016/j.energy.2016.02.079 [21] Zhang W, Li Y, Duan L, et al. Attrition behavior of calcium-based waste during CO2 capture cycles using calcium looping in a fluidized bed reactor[J]. Chemical Engineering Research and Design, 2016, 109: 806-815. doi: 10.1016/j.cherd.2016.04.003 [22] Symonds R T, Champagne S, Ridha F N, et al. CO2 capture performance of CaO-based pellets in a 0.1 MWth pilot-scale calcium looping system[J]. Powder Technology, 2016, 290: 124-131. doi: 10.1016/j.powtec.2015.08.044 [23] Diego M E, Alonso M.Operational feasibility of biomass combustion with in situ CO2 capture by CaO during 360h in a 300 kWth calcium looping facility[J]. Fuel, 2016, 181: 325-329. doi: 10.1016/j.fuel.2016.04.128 [24] Shadle L J, Monazam E R, Swanson M L.Coal gasification in a transport reactor[J]. Industrial & Engineering Chemistry Research, 2001, 40(13): 2782-2792. doi: 10.1021/ie001113u [25] 王圣典. 密相输运床气固流动特性研究[D]. 北京: 中国科学院研究生院, 2012. [26] Souza-Santos M L D.Solid fuels combustion and gasification-modeling, simulation, and equipment operations[M]. Second edition.Boca Raton, London, New York: CRC Press Taylor & Francis Group, 2010. [27] Merrick D.Mathematical models of the thermal decomposition of coal 1. the evolution of volatile matter[J]. Fuel, 1983, 62: 534-539. doi: 10.1016/0016-2361(83)90222-3 [28] Merrick D.Mathematical models of the thermal decomposition of coal 2. Specific heats and heats of reaction[J]. Fuel, 1983, 62: 540-546. doi: 10.1016/0016-2361(83)90223-5 [29] Merrick D.Mathematical models of the thermal decomposition of coal 3. density, porosity and contraction behaviour[J]. Fuel, 1983, 62: 547-552. doi: 10.1016/0016-2361(83)90224-7 [30] 吴学成, 王勤辉, 骆仲泱, 等. 气化参数影响气流床煤气化的模型研究(Ⅰ)-模型建立及验证[J]. 浙江大学学报: 工学版, 2004, 38(10): 1361-1365, 1386. doi: 10.3785/j.issn.1008-973X.2004.10.024Wu Xuecheng, Wang Qinhui, Luo Zhangyang, et al. Modelling on effects of operation parameters on entrained flow coal gasification(Ⅰ): Model established and validation[J]. Journal of Zhejiang University: Engineering Science, 2004, 38(10): 1361-1365, 1386. doi: 10.3785/j.issn.1008-973X.2004.10.024 [31] Liu G S, Niksa S.Coal conversion submodels for design applications at elevated pressures. Part Ⅱ. char gasification[J]. Progress in Energy and Combustion Science, 2004, 30(6): 679-717. doi: 10.1016/j.pecs.2004.08.001 [32] 关键. 新型近零排放煤气化燃烧集成利用系统的机理研究[D]. 杭州: 浙江大学, 2007. -
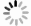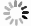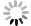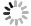





 下载:
下载: